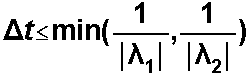The first-order irreversible rate reaction term represents the mass loss of both the dissolved phase and the sorbed phase.
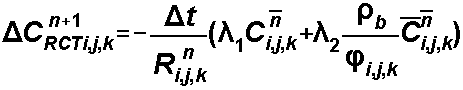
The rate constants are
For radioactive decay, the reaction generally occurs at the same rate in both phases.
For biodegradation some reactions occur only in the dissolved phase
The sorbed concentration is determined by the sorption isotherm
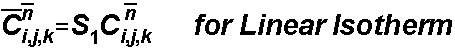
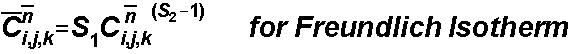
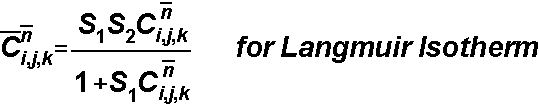
The decay term is solved in Eulerian step, so the stability criterion is: