Furtak Research Projects
Photoactive Monolayers
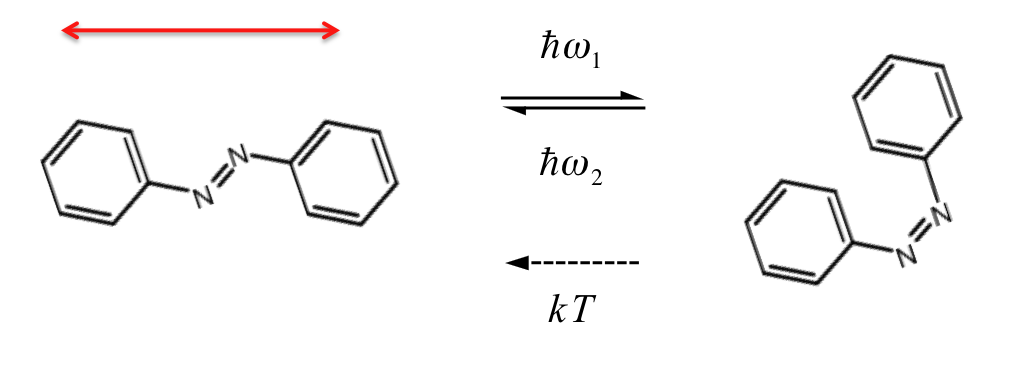 We use self-assembled monolayers (SAMs) containing an azobenzene unit. When exposed to polarized (red arrow) light, the azobenzene undergoes a transformation to the bent (cis) conformation, followed by relaxation back to the straight (trans) form. If this results in a rotation away from the direction of the treatment polarization, then the probability for additional trans-cis-trans motion is reduced. Eventually a significant fraction of the molecules in the sample are aligned with their long axis perpendicular to the treatment polarization.
We use self-assembled monolayers (SAMs) containing an azobenzene unit. When exposed to polarized (red arrow) light, the azobenzene undergoes a transformation to the bent (cis) conformation, followed by relaxation back to the straight (trans) form. If this results in a rotation away from the direction of the treatment polarization, then the probability for additional trans-cis-trans motion is reduced. Eventually a significant fraction of the molecules in the sample are aligned with their long axis perpendicular to the treatment polarization.
This phenomenon can be used to produce a remotely addressable in-plane surface anisotropy for controlling the alignment of liquid crystals for display purposes. Advanced ferroelectric LCs need strong anchoring (alignment synchronization) during cell assembly, but weak anchoring in cell operation. The photoisomerization can also change the wetting properties of structured surfaces. This could be applied to move microdrops or cells by using a gradient in illumination intensity or wavelength.
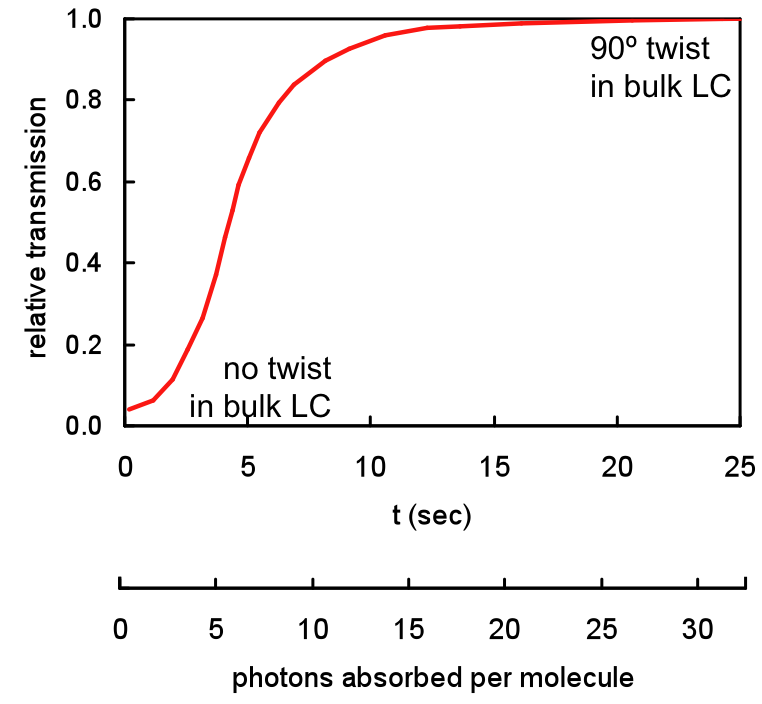 Using a molecule that is similar to methyl red, but with a triethoxysilane branch (derivatized methyl red, dMR), we have produced photo-active SAMs that switch a 20-micron nematic liquid crystal cell in less than 5 seconds using very low levels of polarized treatment radiation. The graph shows the transmission through a test cell containing the dMR monolayer on the interior of one of the cell windows.
Using a molecule that is similar to methyl red, but with a triethoxysilane branch (derivatized methyl red, dMR), we have produced photo-active SAMs that switch a 20-micron nematic liquid crystal cell in less than 5 seconds using very low levels of polarized treatment radiation. The graph shows the transmission through a test cell containing the dMR monolayer on the interior of one of the cell windows.
We are studying the basic properties of dMR, including its very interesting collective effects, using high sensitivity optical probes of the monolayers. The work is conducted by grad students An Lo, Kevin Wood, and Stefan Nikodemski under the Liquid Crystal Materials Research Center, in collaboration with Noel Clark, Dave Walba, Joe McClennan, Youngwoo Yi, and Guanjiu Fang, all at the University of Colorado. Reuben Collins, at Mines, is also a partner in some of the studies.
