Furtak Research Projects
Photoelectrochemistry on Dye-Sensitized Surfaces
One of the most attractive strategies to produce renewable fuel is to use the energy in solar radiation to generate hydrogen from water. This can be accomplished in an electrochemical system where one electrode is a wide bandgap semiconductor. To utilize the available spectrum coming from the sun, it is necessary to "sensitize" the surface of the semiconductor with a layeer that will absorb lower energy radiation. The goal of this research is to develop a stable, cost effective, photo-sensitizers based on polyoxometallates (POMs) to assist large band gap semiconductors for water splitting under visible light irradiation.
Polyoxometallates are a large class of inorganic transition metal oxygen clusters with many unique properties.
- They can be regarded as very small semiconductor quantum dots.
- By varying the transition metal type and position, essentially every molecular property can be altered.
- They have a wide range of transition energies for the first excited state.
- The range of reduction/oxidation potentials is large
- They adsorb very strongly to metals and to metal sites on metal oxides
- They have favorable electron transport and charge separation properties
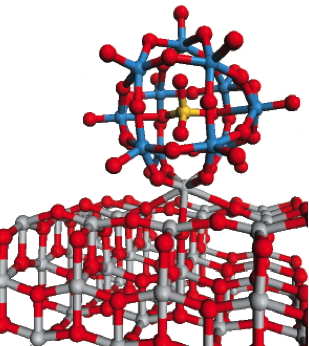 This ball-and-stick diagram, based on density-functional calculations performed by Mark Lusk, shows how a POM molecule with an open cage structure bonds very strongly with a metal oxide surface (titanium dioxide). The model predicts that the open-caged [SiW11O39]8- will pull a Ti atom into the hole in the structure to approximately recreate the closed structure. This type of interaction is expected to enhance charge transfer between the POM and the metal oxide conduction band, thus increasing photocurrent.
This ball-and-stick diagram, based on density-functional calculations performed by Mark Lusk, shows how a POM molecule with an open cage structure bonds very strongly with a metal oxide surface (titanium dioxide). The model predicts that the open-caged [SiW11O39]8- will pull a Ti atom into the hole in the structure to approximately recreate the closed structure. This type of interaction is expected to enhance charge transfer between the POM and the metal oxide conduction band, thus increasing photocurrent.
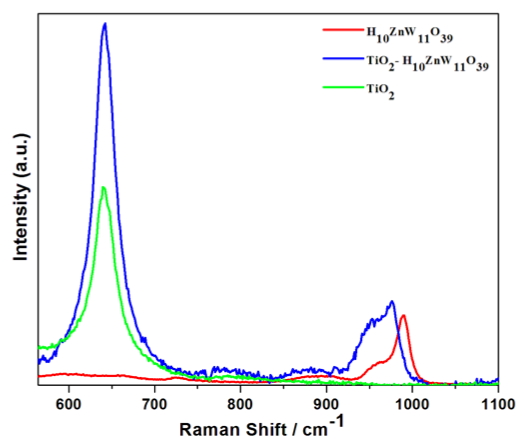 We are using Raman spectroscopy to characterize the POM-metal oxide complex. We use nanocrystalline TiO2 with and without the adsorbed molecule. In the data shown here how H10ZnW11O39 interacts with the oxide. The vibrational features between 900 and 100 wavenumbers are caused by W-O stretching modes associated with the open end of the cage. When the molecule is adsorbed on the oxide these modes shift to lower energy, and indication of bonding perturbation. This signal comes from a single monolayer. So, charge-transfer enhancement of the Raman cross section is necessarily present. This indicates that the alignment of energy levels between this molecule and the oxide are favorable for photoelectrochemistry, where excited state electrons in the POM should transfer to the conduction band of the oxide.
We are using Raman spectroscopy to characterize the POM-metal oxide complex. We use nanocrystalline TiO2 with and without the adsorbed molecule. In the data shown here how H10ZnW11O39 interacts with the oxide. The vibrational features between 900 and 100 wavenumbers are caused by W-O stretching modes associated with the open end of the cage. When the molecule is adsorbed on the oxide these modes shift to lower energy, and indication of bonding perturbation. This signal comes from a single monolayer. So, charge-transfer enhancement of the Raman cross section is necessarily present. This indicates that the alignment of energy levels between this molecule and the oxide are favorable for photoelectrochemistry, where excited state electrons in the POM should transfer to the conduction band of the oxide.
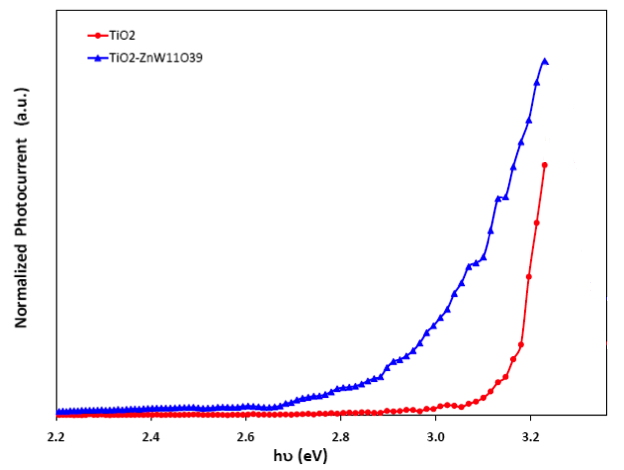 The last figure shows the photoelectrochemical action spectrum of a nanoporous TiO2 electrode. The blue curve is for the sensitized surface. The modified electrode absorbs light in the visible range of the optical spectrum. While this result is similar to sensitized photoelectrochemistry phenomena that have been documented for decades, the range of chemical design flexibility afforded by the POM system could lead to much higher solar conversion efficiencies.
The last figure shows the photoelectrochemical action spectrum of a nanoporous TiO2 electrode. The blue curve is for the sensitized surface. The modified electrode absorbs light in the visible range of the optical spectrum. While this result is similar to sensitized photoelectrochemistry phenomena that have been documented for decades, the range of chemical design flexibility afforded by the POM system could lead to much higher solar conversion efficiencies.
In addition to Mark Lusk, the photoelectrochemical project is a collaboration with Andy Herring (Chemical Engineering) and John Turner (NREL). Much of the work was performed by Postdoctoral Fellow Pilli Satyananda Kishore. Sponsorship is through the Center for Revolutionary Solar Photoconversion.
