Publications and Presentations
Steven C. DeCaluwe
Archival Publications
2020
-
Stetson, C.; Yin, Y.; Norman, A.; Harvey, S.P.; Schnabel, M.; Ban, C.; Jiang, C.S.; DeCaluwe, S.C.; Al-Jassim, M. "Evolution of Solid Electrolyte Interphase and Active Material in the Silicon Wafer Model System." J. Power Sources. 482(15), 2020, 228946.
Lay Summary: Silicon-based anodes are promising for lithium-ion batteries, due to their ability hold more energy per unit mass, compared to conventional technology. However, bringing this technology to market is difficult due to degradation of the anode. This degradation is exaccerbated by an unstable solid electrolyte interphase (aka 'sei'), a layer that helps prolong battery lifetime. It is very difficult to learn direct about the SEI, because it is very thin (10s of nanometers), delicate, and chemically sensitive, which makes it difficult to conduct measurements of unaltered SEI layers.
In this paper, we study the evolution of the SEI on a simplified thin-film silicon anode, using a variety of techniques to understand the changes to its chemical, structural, and electronic properties during battery use. The results add to the growing evidence that the SEI on silicon anodes is a dynamic, constantly-changing entity, and that changes in the SEI are linked to changes in the underlying silicon active material.
-
Baker, J.A.; Beuse, M.; DeCaluwe, S.C.; Jing, L.W.; Khoo, E.; Sripad, S.; Ulissi, U.; Verma, A.; Wang, A.A.; Yeh, Y.T.; Yiu, N.; Howey, D.A.; Viswanathan, V. "Fostering a Sustainable Community in Batteries." ACS Energy Lett. 5, 2020, 2361 - 2366. Citations: 2
 Open Access: Download the Open Access Article Here
Open Access: Download the Open Access Article Here Lay Summary: In the wake of the SARS-cov-2 pandemic, researchers of multiple stripes have found creative ways to replace the in-person networking events. This article describes a new online community that has emerged broadly focused on battery modeling. The online community includes a weekly battery modeling seminar series and open discourse via Slack. The article describes the origins and initial activities of the community, and gives a compelling argument for the benefits of such online communities, in terms of environmental impact, inclusivity, and the free exchange of ideas.
-
Randall, C.R.; DeCaluwe, S.C. "Physically Based Modeling of PEMFC Cathode Catalyst Layers: Effective Microstructure and Ionomer Structure-Property Relationship Impacts." J. Electrochem. Energy. Conv. Storage: 2020 Emerging Investigators Special Issue, 17(4), 2020, 041104 - 041113.
 Open Access: Download the Open Access Article Here
Open Access: Download the Open Access Article HereLay Summary: Polymer Electrolyte Membrane Fuel Cells (PEMFCs) convert chemical energy in fuels directly into electrical energy. If the fuel is hydrogen, heat and water are the only waste products of the fuel cell. As such, PEMFCs offer considerable promise as part of a clean-energy future based on hydrogen fuel. However, among other challenges, manufacturers must reduce the amount of the expensive platinum catalyst current used in PEMFCs. At present, decreasing the Pt to DOE benchmarks leads to unacceptable and unexplained losses in fuel cell performance and efficiency.
In this paper, we use a numerical simulation of PEMFC performance to explore the impact of the physical microstructure in the PEMFC and the properties of the ion-conducting polymer Nafion on PEMFC performance with low platinum loading. Previous work published by our group reveals changes to the structure and transport properties of Nafion with decreasing polymer thickness. In this work, we incorporate these structure-property relationships into physically-informed microstructure models of the PEMFC to demonstrate the such relationship can in fact help explain the losses seen at low Pt loading. Results also suggest that an optimal Nafion thickness may exist that balances the competing transport of reactants (oxygen) and charge (H+ ions), guiding future PEMFC design and development efforts.
2019
- Khoo, E.; Lacey, M.J.; DeCaluwe, S.C. "Social Media Platforms for Electrochemistry." ECS Interface, 28(4), 2019, 41. Citations: 1
 Open Access: Download Article Here
Open Access: Download Article Here Lay Summary:
Increasingly, social media platforms such as Twitter, LinkedIn, and Reddit provide informal networking opportunities for electrochemistry researchers. These avenues have low barrier for entry, and facilitate the exchange of ideas, establishing professional connections, and building a sense of community. In this article, we describe the current state and the benefits of social media for electrochemistry researchers. - Stetson, C.; Yin, Y.; Jiang, C.-S.; DeCaluwe, S.C.; Al-Jassim, M.; Neale, N.R.; Ban, C.; Burrell, A. "Temperature-Dependent Solubility of Solid Electrolyte Interphase on Silicon Electrodes." ACS Energy Lett., 4, 2019, 2770-2775. Citations: 3
Lay Summary:
The solid electrolyte interphase (SEI) is a layer that forms in batteries due to materials degradation. Once formed, the SEI serves a protective function to prevent further breakdown. However, long-term instability of the SEI is a major impediment to durable and efficient batteries for a range of energy storage applications. In batteries with silicon anodes, studying the SEI is complicated because the voltages where the SEI forms overlap with those where lithium inserts into the silicon anode, leading to large swelling of the anode which changes the SEI structure.In this paper, we demonstrate the use of a high cutoff potential to study the SEI that is formed prior to silicon anode swelling. We use an AFM-based microscope technique (scanning-spreading resistance microscopy, SSRM) to study the SEI thickness, surface roughness, and resistance (i.e. how easily it conducts electrons) after it is formed and after it rests at temperatures ranging from -10 to 50 °C. We find that the SEI becomes smoother, thinner, and less resistive after resting, due to re-dissolution of SEI components. The dissolution rates and corresponding properties are amplified by rests at higher temperatures - the SEI is smoothest, thinnest, and least resistive after resting at 50 °C. These results demonstrate a method to evaluate SEI properties for any number of modifications proposed to improve the SEI's durability in operating batteries.
- Mayur. M; DeCaluwe, S.C.; Kee, B.L.; Bessler, W.G. "Modeling and simulation of the thermodynamics of lithium-ion battery intercalation materials in the open-source software Cantera." Electrochimica Acta, 323, 2019, p. 134797. Citations: 1
 Open Access: Download Article Here
Open Access: Download Article Here Lay Summary: The electrochemical behavior in lithium-ion batteries can be quite complex. Lithium-ion batteries rely on a process called intercalation, where lithium is inserted into the host material, which lead to a change in the enthalpy and entropy of the host material. The thermodynamics of these electrochemical processes have been studied experimentally for specific materials, but have yet to be implemented into computational tools in this scope. Cantera is an open-source software simulation toolkit that provides a platform for modeling complex reaction system based on underlying thermodynamics, kinetics, and transport. A new Cantera thermodynamics class named BinarySolutionTabulatedThermo has been implemented in this work. With this class, tabulated concentration-dependent molar enthalpies and entropies are the inputs instead of empirical analytical expression. This method provides better flexibility for switching between materials, a more fundamental representation of the material properties, and a more precise parameterization from experimental half-cell data.
-
Kee, B.L.; Curran, D.; Zhu, H.; Braun, R.J.; DeCaluwe, S.C.; Kee, R.J.; Ricote, S. "Thermodynamic Insights for Electrochemical Hydrogen Compression with Proton-Conducting Membranes." Membranes, 9, 2019. p. 134797. Citations: 2
 Open Access: Download Article Here
Open Access: Download Article Here Lay Summary: The production of high pressure hydrogen is an increasingly growing market. Currently, the majority of compressed hydrogen is produced from steam methane reforming, separated in pressure swing adsorption beds, then compressed in mechanical-based compressors. Electrochemical hydrogen compression is an attractive alternative to mechanical compression due to the lack of moving parts, contamination issues, and maintenance costs. Polymer-electrolyte membranes are a relatively mature electrochemical technology and operate at temperatures less than 100 °C. Protonic-ceramic membranes are a relatively new electrochemical technology that requires high temperatures near 600 °C and offer potential efficiency enhancements over the typical hydrogen production methods. Protonic-ceramics are very attractive because they can beneficially combine hydrogen production, separation, and compression.
Using a thermodynamics-based analysis, this paper explores the technology implications for these two electrochemical membranes. Temperature, pressure, work applied, and heat management all play significant roles in choosing the appropriate technology. The results suggest significant benefits associated with systems that combine protonic-ceramic reactors to reform fuels and deliver lightly compressed hydrogen (near 5 bar) to an electrochemical compressor using a polymer electrolyte membrane to compress to very high pressure.
-
DeCaluwe, S.C., "Open software for chemical and electrochemical modeling: Opportunities and challenges." ECS Interface, 28, 2019, p. 47—50. Citations: 1
 Open Access: Download Article Here.
Open Access: Download Article Here.Lay Summary: Electrochemistry plays an essential role in any number of next-generation technologies, ranging from energy conversion and storage (i.e. batteries and fuel cells), to chemical and fuels processing, to sensors and detectors. To meet consumer demands, device designers must commonly provide precise control over increasingly complex electrochemistry.
Modeling and simulation of electrochemical systems can play an essential role in enabling this precise control, but require new approaches and tools. In this "thought leadership" article, we discuss some of the new open source modeling and simulation tools ("open source" = free for all to use and edit) available for electrochemical modeling. These tools allow modelers to flexibly and efficiently implement electrochemical models with varying complexity, and also provide tools for efficiently developing, maintaining, and sharing software tools in a collaborative research environment. Lastly, we discuss challenges which must be addressed, in order to support the efficient development and widespread use of electrochemical modeling tools, going forward.
-
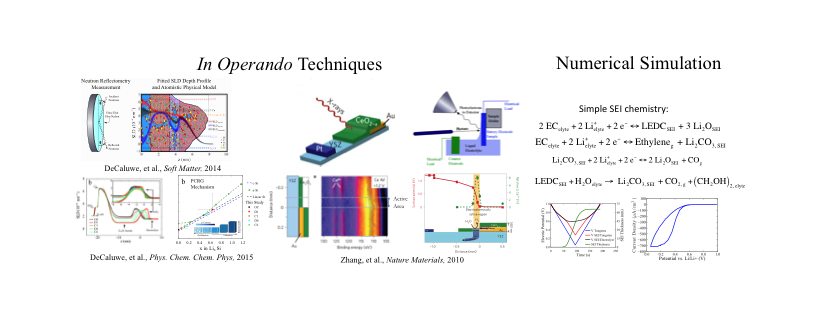
Lee, C.H., Dura, J.A., LeBar, A., DeCaluwe, S.C., "Direct, operando observation of the bilayer solid electrolyte interphase structure: Electrolyte reduction on a non-intercalating electrode." J. Power Sources, 412, 2019, p. 725—735. Citations: 7
Download Preprint Download Supplementary Information
Lay Summary: The solid electrolyte interphase (SEI) is a layer that forms in batteries due to materials degradation. Once formed, the SEI serves a protective function to prevent further breakdown. However, long-term instability of the SEI is a major impediment to durable and efficient batteries for a range of energy storage applications. In part, engineering a better, more stable SEI is challenging due to limited fundamental information about the chemistry involved in its formation. The SEI is a very thin (10-100 nm) layer and is very sensitive to the local chemical environment, making it very difficult to measure unaltered SEI layers under relevant conditions.
In this paper, we use neutron reflectometry (NR) and quartz crystal microbalance (QCM) to study the SEI thickness, structure, and chemistry in the chemical environment in which it is grown. NR can measure the thickness and provides basic chemical composition information for very thin films, and QCM is a very sensitive mass balance (ng/cm2), to provide two complementary measures of an SEI grown in a very simple battery. By isolating the SEI, we learn new things about its structure, chemical makeup, and the way it forms, which can provide insights to make stable and efficient batteries, in the future.
2018
-
DeCaluwe, S.C., Weddle, P.J, Zhu, H.Y., Colclasure, A.M., Bessler, W.G., Jackson, G.S., Kee, R.J., "On the Fundamental and Practical Aspects of Modeling Complex Electrochemical Kinetics and Transport." J. Electrochem. Soc., 165, 2018, p. E637—E658. Citations: 8.
Lay Summary: Electrochemical technologies, such as fuel cells, batteries, and sensors, play important roles in many areas of life with a wide range of benefits to society. Computer simulations can be a powerful and efficient way to identify new designs and chemistries for improved devices. However, at present, there exist no general-purpose tools for modeling complex electrochemical phenomena, which therefore slows the pace of development for potentially beneficial technologies.
In this article, we provide several examples of 'electrochemical complexity' in a range of different devices, and give a perspective on the modeling capabilities required to describe complex chemical reactions and molecular transport processes in a generalized manner. It is important that these capabilities are implemented in a way such that they can be employed broadly across a range of complex phenomena and devices. We then provide an example of how such tools could be deployed within a generalized software framework, using the Cantera software package as a framework.
-
DeCaluwe, S.C., Baker, A.M., Bhargava, P., Fischer, J.E., Dura, J.A., "Structure-property relationships at Nafion thin-film interfaces: Thickness effects on hydration and anisotropic ion transport." Nano Energy, 46, 2018, p. 91—100. Citations: 32.
Download Preprint Here. Download Supplementary Information Here.
Lay Summary: The polymer Nafion is used in low-temperature fuel cells used in electric fuel cell vehicles (which run on hydrogen fuel and produce water as the only exhaust). In this paper, we study what happens to the Nafion properties when the polymer films get very thin (< 100 nm thick).
We show that the way Nafion interacts with nearby materials can affect how the polymer's molecules arrange themselves. When the polymer gets very thin, this impacts how much water the polymer can absorb and how it is able to move hydrogen ions to and from the fuel cell electrochemical reaction sites.
-
Kogekar, G., Karakaya, C., Liskovich, G.J., Oehlschlaeger, M.A., DeCaluwe, S.C., Kee, R.J., "Impact of non-ideal behavior on ignition delay and chemical kinetics in high-pressure shock tube reactors." Combustion and Flame, 189, 2018, p. 1—11. Citations: 11.
Lay Summary: An equation of state is a mathematical formula (or set of formulae) that describe the behavior of chemical substances (solids liquids and gases). The ideal gas law is one commonly-known example. Here, we explore the impact of the equation of state (ideal gas law vs. a more accurate equation) on how the fuel n-dodecane combusts at high pressures.
We find that the equation of state has a relatively small impact on the density during combustion of n-dodecane at up to 100 times atmospheric pressure. However, the equation of state has a significant impact on the reactivity of the species involved (i.e., the species activities), and this must be correctly incorporated for accurate combustion predictions.
2017
-
Kee, B., Karakaya, C., Zhu, H. DeCaluwe, S.C., Kee, R.J., "The influence of hydrogen-permeable membranes and pressure on methane dehydroaromatization in packed-bed catalytic reactors." Ind. Eng. Chem. Res., 56(13), 2017, p. 3551—3559. Citations: 7.
Lay Summary: Methane Dehydroaromatization (MDA) is a way to turn cheap and plentiful methane into more valuable fuels, such as benzene. Here, we explore two possible methods to make MDA more efficient: use of a membrane to separate out reaction products (H2), and operation at high pressures.
We find that hydrogen removal increases the rate at which methane is converted, but most of this increased conversion goes toward production of unwanted and harmful byproducts. Increasing the pressure decreases the methane conversion rate, but increases benzene prodution rates. This may be a viable path toward benzene production with more complicated reactor designs.
2015
-
DeCaluwe, S.C., Dhar, B.M., Huang, L., He, Y., Yang, K., Owejan, P., Zhao, Y., Talin, A.A., Dura, J.A., Wang, H., "Pore collapse and regrowth in silicon electrodes for rechargeable batteries." Phys. Chem. Chem. Phys., 17(17), 2015, p. 11301—11312. Citations: 20.
Download Preprint Here. Download Supplementary Information Here.
Lay Summary: Silicon is a promising material for rechargeable lithium batteries, as it can store significantly more energy per unit mass than current battery materials. However, it is not very durable, due to expansion and contraction during battery operation. Here, we use neutrons to study thin-film silicon anodes to better understand how it expands and contracts during battery cycling.
We observe that small pores in the silicon film collapse and regrow during battery operation, acting as a sort of 'reservoir' to absorb some of the silicon volume expansion. This seems to help prevent the catastrophic breakup of the electrode, although minor degradation is still observed. These pores also help explain the volume expansion trends in this study and in several previous studies, which had not fully matched with predictions.
2014
-
DeCaluwe, S.C., Kienzle, P.A., Bhargava, P., Baker, A.M., Dura, J.A., "Phase segregation of sulfonate groups in Nafion interface lamellae, quantified via neutron reflectometry fitting techniques for multi-layered structures." Soft Matt., 10(31), 2014, p. 5763—5776. Citations: 55.
Download Preprint Here. Download Supplementary Information Here.
Lay Summary: The polymer Nafion is used in low-temperature (PEM) fuel cells used in electric fuel cell vehicles (which run on hydrogen fuel and produce water as the only exhuast). In this paper, we study very thin Nafion films (< 50 nm), similar to the thickness of the Nafion where many important fuel cell limitations occur.
We confirm that the Nafion molecules spontaneously form sheet-like lamellae, and use the neutron measurements for a very accurate, quantitative description of this arrangement. This helps us understand movement of different chemical species (oxygen, hydrogen, water) and specifically how the Nafion binds to the solid surface, which can help future researchers make more durable or efficient fuel cells, in the future.
2012
-
Zhang, C.J., Grass, M.E., Yu, Y., Gaskell, K.J., DeCaluwe, S.C., Chang, R., Jackson, G.S., Hussain, Z., Bluhm, H., Eichhorn, B.W., Liu, Z., "Multielement activity mapping and potential mapping in solid oxide electrochemical cells through the use of operando XPS." ACS Catal., 2(11), 2012, p. 2297—2304. Citations: 59.
Lay Summary: Solid Oxide Electrochemical Cells (SOECs) are high-temperature devices which convert energy between electrical and chemical (fuel + oxygen) forms. Here, we use a novel technique called Ambient Pressure XPS (AP-XPS) to understand the chemical reactions happening at the surfaces of some SOFC materials. XPS usually occurs at room temperature and under a vacuum, but AP-XPS can study material surfaces at higher temperatures and in relevant chemical environments.
By tracking surface concentrations and electric potentials of active and inactive species, we are able to identify the electrochemically active region of the CeO2-x anode catalyst and directly evaluate the various surface overpotentials which contribute to performance losses in the SOECs.
-
Eastman, S.A., Kim, S., Page, K.A., Rowe, B.W., Kang, S.H., DeCaluwe, S.C., Dura, J.A., Soles, C.L., Yager, K.G., "Effect of confinement on structure, water solubility, and water transport in Nafion thin films." Macromol., 45(19), 2012, p. 7920—7930. Citations: 148.
Lay Summary: The polymer Nafion is used in low-temperature fuel cells used in electric fuel cell vehicles (which run on hydrogen fuel and produce water as the only exhuast). In this paper, we study what hapens to the Nafion properties (structure, ability to absorb water, and speed at which water can move inside the polymer [i.e. the water mobility]) when the polymer films get very thin (< 60 nm thick).
We find that the polymer properties are mostly constant for films thicker than 60 nm, but ability to absorb water and the water mobility both decrease with decreasing thickness below 60 nm.
-
Owejan, J.E., Owejan, J.P., DeCaluwe, S.C., Dura, J.A., "Solid electrolyte interphase in Li-ion batteries: Evolving structures measured in situ by neutron reflectometry." Chem. Mat., 24(11), 2012, p. 2133—2140. Citations: 121.
Download Preprint Here. Download Supplementary Information Here.
Lay Summary: Advances in Li-ion batteries have enabled a range of energy storage applications for portable devices and intermittent renewable energy sources alike. One of the central remaining challenges in Li-ion batteries regards the solid electrolyte interphase (SEI), a protective layer in the battery that helps prevent breakdown of the battery materials. Improving this layer would lead to lighter and more durable batteries, but studying it is very difficult, because it is a very thin, chemically sensitive layer, buried inside the battery structure.
Here, we use neutron scattering as a means to directly measure SEI thickness, porosity, and chemical composition during its growth. We find that the SEI is initially 4.0 nm thick, and grows thicker with addittional battery operation. We also find that the SEI evolves dynamically during the charging and discharging of the battery. Deposited materials are not permanently 'locked' in the SEI; rather, some components are deposited and re-dissolved continuously during battery operation.
2010
-
DeCaluwe, S.C., Grass, M.E., Zhang, C.J., El Gabaly, F., Bluhm, H., Liu, Z., Jackson, G.S., McDaniel, A.H., McCarty, K.F., Farrow, R.L., Linne, M.A., Hussain, Z., Eichhorn, B.W., "In situ characterization of ceria oxidation states in high-temperature electrochemical cells with ambient pressure XPS." J. Phys. Chem. C, 114(46), 2010, p. 19853—19861. Citations: 96.
Download Preprint Here. Download Supplementary Information Here.
Lay Summary: Solid Oxide Electrochemical Cells (SOECs) are high-temperature devices which convert energy between electrical and chemical (fuel + oxygen) forms. Here, we use ambient pressure XPS (AP-XPS) to understand how the oxidation state of ceria (a catalyst that makes SOECs more tolerant to carbon and sulfur in fuels) changes as a function of operating conditions.
The use of AP-XPS enables new insights into the properties of the ceria surface. First, we see that the surface has much less oxygen than the interior of ceria, likely due to surface strain energy. We also show that the amount of surface oxygen changes significantly during fuel cell operation, demonstrating that slow surface reaction rates limit ceria's performance in SOECs. Finally, we show the impact of mixed conductivity in ceria, as the reactions are able to occur much farther from the current collector (100s of microns) than in conventional SOEC materials.
-
Zhang, C.J., Grass, M.E., McDaniel, A.H., DeCaluwe, S.C., El Gabaly, F., Liu, Z., McCarty, K.F., Farrow, R.L., Linne, M.A., Hussain, Z., Jackson, G.S., Bluhm, H., Eichhorn, B.W., "Measuring fundamental properties in operating solid oxide electrochemical cells by using in situ X-ray photoelectron spectroscopy." Nature Materials, 9(11), 2010, p. 944—949. Citations: 252.
Download Preprint Here. Download Supplementary Information Here.
Lay Summary: Solid Oxide Electrochemical Cells (SOECs) are high-temperature devices which convert energy between electrical and chemical (fuel + oxygen) forms. Here, we demonstrate the first-ever use of ambient pressure XPS (AP-XPS) to study the active surface states in an operating SOEC.
By evaluating the active catalyst and electrolyte surface chemical states and electric potentials, we directly resolve the surface voltahge losses in the SOEC, which directly match with conventional voltage measurements but give more specific information. We also use the change in surface concentrations and surface electric potentials to identify the active surface regions, which in the ceria electrode studied are much larger than in typical SOEC materials (e.g. nickel). Finally, by varying the ceria films thickness, we show that the ceria surface chemistry is a significant limiting factor in SOEC efficiency, with faster chemistry and higher efficiency found in thicker films.
2008
-
DeCaluwe, S.C., Zhu, H., Kee, R.J., Jackson, G.S., "Importance of anode microstructure in modeling solid oxide fuel cells." J. Electrochem. Soc., 155(6), 2008, p. B538—B546. Citations: 61.
Lay Summary: This paper looks at the importance of microstructure in modeling solid oxide fuel cell (SOFC) operation. SOFCs are high-temperature devices which convert chemical energy (fuel + air) to electrical energy. Here, we build a one-dimensional SOFC numerical model, and use it to understand the importance of various microstructural parameters in predicting SOFC performance.
By comparing our simulations to previously published experimentals, we reach several key findings. First, we uncover a common error in porous media transport modeling, which persists back to the early 20th century. After correctly incorporating the tortuosity into transport calculations, we also find that the resistances due to gas transport, surface chemical reactions, and electrolyte-phase ion conduction are interrelated. For operating conditions where gas transport is limiting, these resistances cannot be calculated independent of one another.