Next Generation Battery Materials
 Lithium ion batteries (LIBs) currently dominate the market and are expected to for another decade as costs decline.
However, with performance approaching intrinsic material limits, LIBs cannot meet the increasing demands of electric vehicles,
advanced consumer electronics, and stationary storage. In the next decade both solid state and lithium-sulfur (Li-S) batteries
are expected to start displacing LIBs, and the market for these alternatives is forecast to be over $30 billion by 2035.
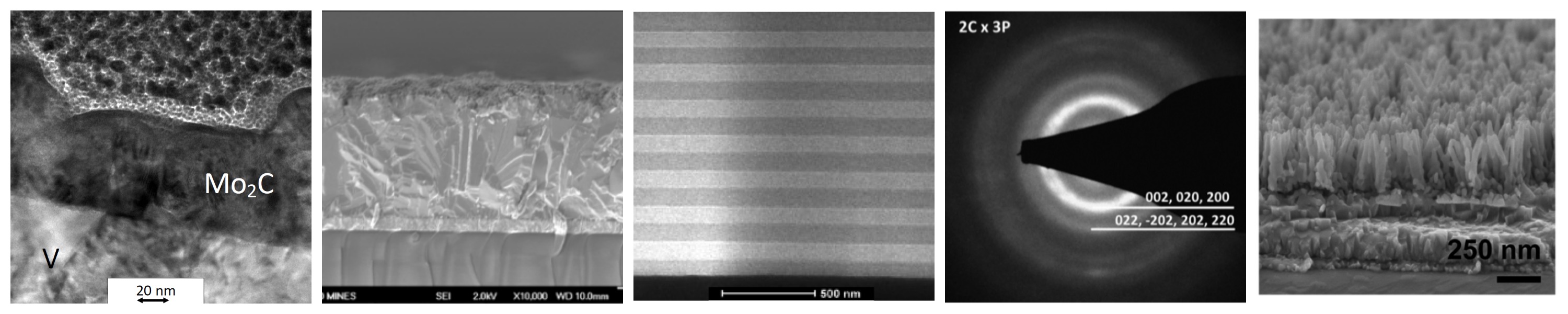
Central to both technologies is Li2S, which serves as the active cathode material in Li-S batteries and is the key precursor
and cost driver for leading solid-state electrolytes. In both applications Li2S has demonstrated excellent performance when used
in nanoparticle form. However, Li2S is very costly and commercially available only as micropowders with impurities being a
major concern, reflecting the energy-intensive carbothermal reduction processes currently used for synthesis. The sodium analogue (Na2S) is
also of interest for low cost, stationary storage applications.
Lithium ion batteries (LIBs) currently dominate the market and are expected to for another decade as costs decline.
However, with performance approaching intrinsic material limits, LIBs cannot meet the increasing demands of electric vehicles,
advanced consumer electronics, and stationary storage. In the next decade both solid state and lithium-sulfur (Li-S) batteries
are expected to start displacing LIBs, and the market for these alternatives is forecast to be over $30 billion by 2035.
Central to both technologies is Li2S, which serves as the active cathode material in Li-S batteries and is the key precursor
and cost driver for leading solid-state electrolytes. In both applications Li2S has demonstrated excellent performance when used
in nanoparticle form. However, Li2S is very costly and commercially available only as micropowders with impurities being a
major concern, reflecting the energy-intensive carbothermal reduction processes currently used for synthesis. The sodium analogue (Na2S) is
also of interest for low cost, stationary storage applications.
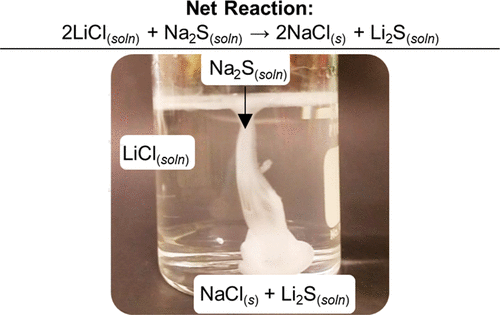
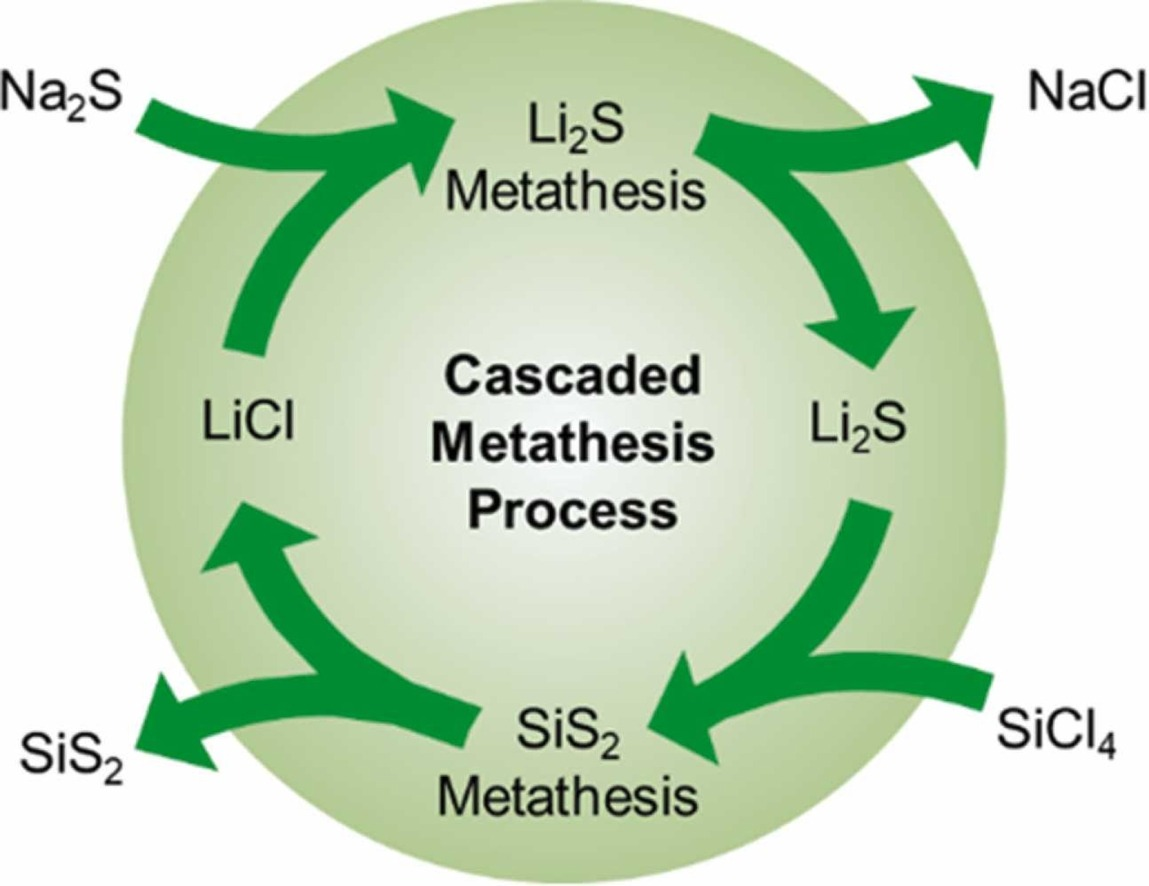 We
recently invented a green chemistry-inspired approach for synthesizing lithium sulfide (Li2S) nanocrystals through
a simple metathesis between LiCl and Na2S as shown above. The reaction is instantaneous and goes to completion, driven
by precipitation of NaCl. Moreover this Li2S may be used in a subsequent metathesis to produce a new sulfie
(in this case SiS2 and regenerating the valuable LiCl. This process was develop by recent PhD grad Will Smith and
development continues with current PhD candidate Saeed Ahmadi Vaselabadi and UG assistant Brynn Benham. In addition we
are developing solution based approached for the ternary and quaternary sodium chalcogenides for advanced battery storage.
We
recently invented a green chemistry-inspired approach for synthesizing lithium sulfide (Li2S) nanocrystals through
a simple metathesis between LiCl and Na2S as shown above. The reaction is instantaneous and goes to completion, driven
by precipitation of NaCl. Moreover this Li2S may be used in a subsequent metathesis to produce a new sulfie
(in this case SiS2 and regenerating the valuable LiCl. This process was develop by recent PhD grad Will Smith and
development continues with current PhD candidate Saeed Ahmadi Vaselabadi and UG assistant Brynn Benham. In addition we
are developing solution based approached for the ternary and quaternary sodium chalcogenides for advanced battery storage.
 This project is supported by the National Science Foundation through award
2219184
and an associated REU supplement.
This project is supported by the National Science Foundation through award
2219184
and an associated REU supplement.